Unmasking Ovarian Cancer: How a “Secret Cell Alliance” Drives Rapid Spread
Ovarian cancer has long been one of oncology’s most formidable challenges — particularly because it often goes undetected until advanced stages and spreads aggressively throughout the abdominal cavity. A new study from Nagoya University now reveals a previously overlooked mechanism that may help explain this stealthy metastatic behavior and open doors to better diagnostics and therapeutic strategies.
Cancer Cells Don’t Go It Alone — They Recruit Help
Traditionally, cancer metastasis has been studied as a process driven by autonomous tumor cells. However, the new research demonstrates that ovarian cancer cells actively enlist normally protective mesothelial cells — the cells lining the abdominal cavity — to create hybrid clusters that accelerate invasion.
What’s especially striking is that these cancer–mesothelial clusters aren’t just passive hitchhikers:
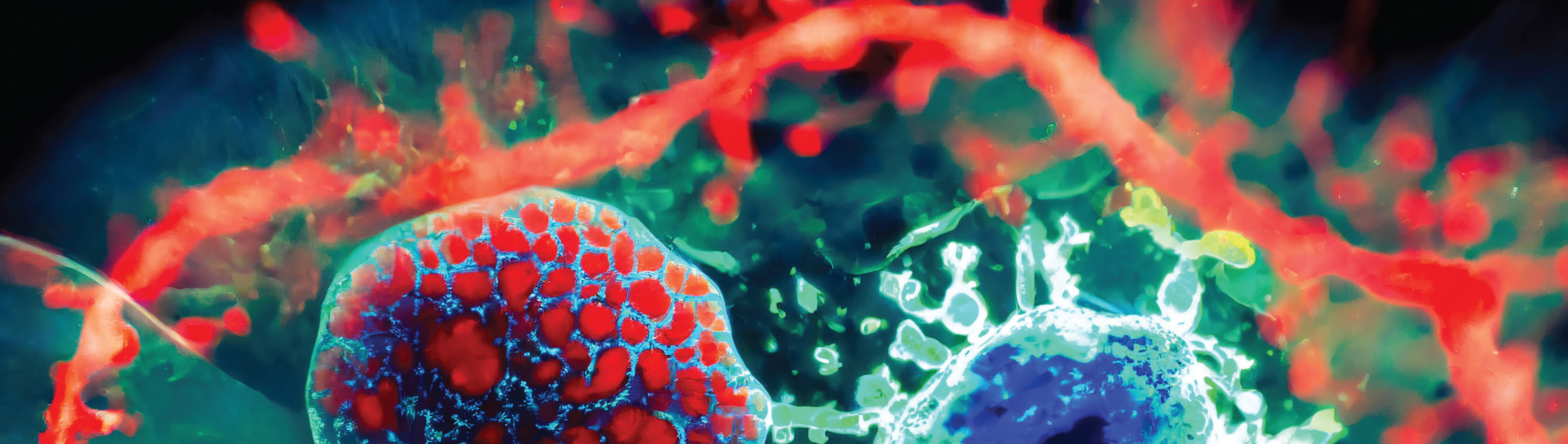
- They form compact, mixed cell spheres in ascitic (abdominal) fluid, with roughly 60% of clusters containing both cancer and mesothelial cells.
- Mesothelial cells, once recruited, develop invadopodia — sharp, actin-rich protrusions that physically cut into surrounding tissues, effectively “leading” the invasion.
- These hybrid spheres are more chemotherapy-resistant than cancer cells alone.
As lead author Dr. Kaname Uno notes, the cancer cells themselves remain genetically similar to their original state. It’s their capacity to manipulate nearby normal cells into collaborators that makes the difference.
“They manipulate mesothelial cells to do the tissue invasion work. They undergo minimal genetic changes and just migrate through the openings those cells create,” according to Dr. Uno.
This discovery reframes our understanding of ovarian metastasis — not merely as rogue cancer cell migration, but as an orchestrated cooperation with host tissues.
What This Means for Research and Clinical Practice
The implications extend across several layers of basic and translational research:
- New Targets for Therapeutics
Blocking the signaling axis that enables cancer cells to recruit and reprogram mesothelial cells could represent a novel approach. For instance, inhibiting TGF-β1 — the molecule shown to trigger mesothelial transformation — may disrupt the formation of hybrid clusters. - Improved Biomarkers for Early Detection
Monitoring co-occurring cell clusters in ascitic fluid, rather than looking for free-floating tumor cells alone, may yield earlier or more accurate indicators of disease progression. - Better Model Systems for Drug Screening
Preclinical models that incorporate tumor–mesothelial cell interactions are likely to be more predictive of therapeutic efficacy — an important consideration for drug development pipelines.
For molecular biologists and biotechnologists, this study underscores the value of single-cell profiling and live-imaging technologies to dissect complex cell–cell interactions in the tumor microenvironment. Procurement professionals and lab managers should take note of the increasing demand for advanced microscopy platforms, single-cell sequencing reagents, and fluid biopsy processing tools capable of identifying rare hybrid cell populations.
Bridging Basic Discovery With Clinical Impact
This work also highlights how a deeper mechanistic understanding of metastatic spread can inform both diagnostics and therapeutic strategy. Unlike many cancers that spread via defined vascular routes, ovarian cancer takes advantage of the relatively unregulated movement of cells within abdominal fluid — and now we know it brings reinforcements to speed the invasion.
For clinicians and diagnostics professionals, the message is clear: monitoring tissue invasion requires tools that can detect complex cellular interactions — not just single tumor cells.
Pro Lab Supply stays close to the science to support laboratories working at the intersection of discovery, diagnostics, and application.
Source:
Nagoya University. “A secret cell alliance may explain why ovarian cancer is so deadly.” ScienceDaily, February 9, 2026. (ScienceDaily)