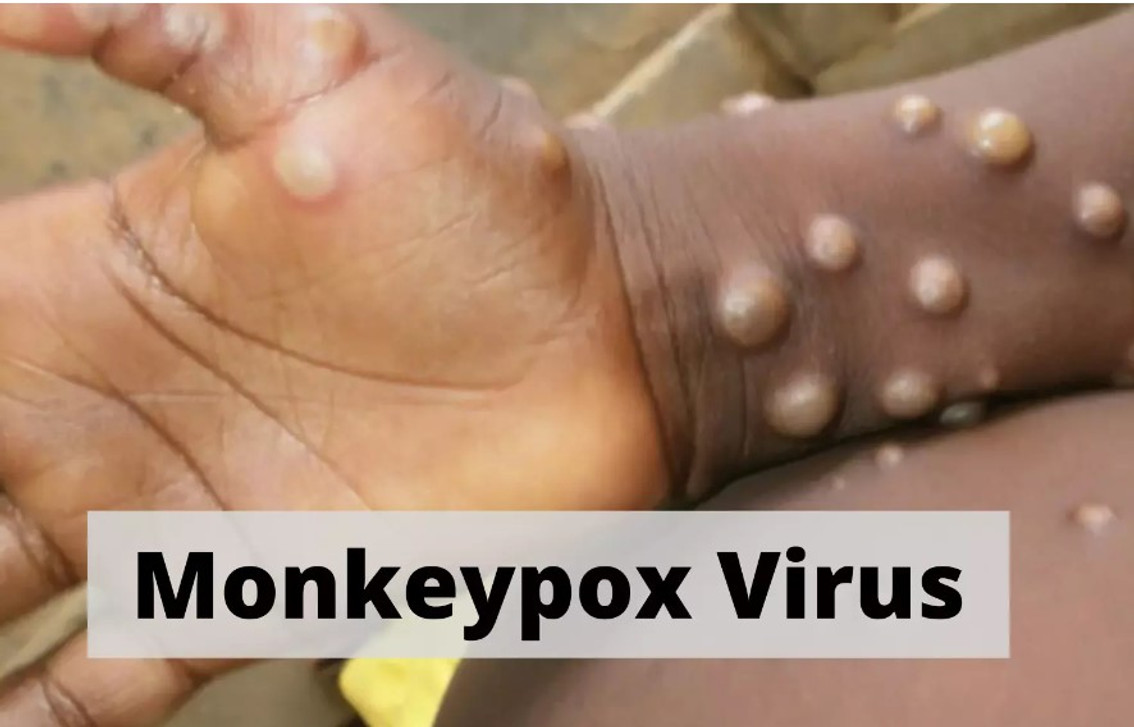
In the United States, the first case of monkeypox in 2022 was diagnosed in a patient hospitalized in Massachusetts who had recently traveled to Canada in private transportation. In 2021, two people traveling from Nigeria to the US were diagnosed with the disease, according to the Centers for Disease Control and Prevention.
According with CDC on June 8 , in USA there are 40 case detected.
| Arizona | 1 |
| California | 8 |
| Colorado | 3 |
| District Of Columbia | 2 |
| Florida | 4 |
| Georgia | 1 |
| Hawaii | 2 |
| Illinois | 3 |
| Massachusetts | 1 |
| New York | 9 |
| Pennsylvania | 1 |
| Texas | 1 |
| Utah | 2 |
| Virginia | 1 |
| Washington | 1 |
Data as of June 8, 2022, 2 pm Eastern. Data will be updated Monday–Friday.
But What is the recommendation for MonkeyPox PCR detection, the CDC recommend the following
Poxvirus Molecular Detection
CDC-10515
Synonym(s)
CDC Pre-Approval Needed
Supplemental Information Required Consultation is required prior to specimen submission. A brief written clinical summary with pertinent medical information (e.g. rash onset date, rash type, symptoms, smallpox vaccination date if relevant) and exposure history should be included. Information must be documented in written form, discussion during initial phone consultation is not a suitable alternative to a written record.
Supplemental Form None
Performed on Specimens from Human and Animal
Acceptable Sample/ Specimen Type for Testing Lesion material is required for persons with an active lesion or rash. A dry swab of lesion fluid or the surface of the lesion is acceptable. If monkeypoxvirus is not suspected, viral culture can also be tested.
Minimum Volume Required 0.5 mL (for viral cultures, if monkeypoxvirus is not suspected)
Collection, Storage, and Preservation of Specimen Prior to Shipping Freeze (-20°C or lower) specimens within an hour after collection. Store frozen samples for up to 60 days. Freezing is strongly recommended. However, if there is no freezer available, refrigerate samples (2-8°C) and store for up to 7 days. Samples must be received within 60 days if frozen or 7 days if refrigerated.
Transport Medium Transport media should not be added to specimens.
Specimen Labeling This test order can accommodate diagnostic and non-diagnostic specimens. Testing subject to CLIA regulations requires two primary patient identifiers (e.g., patient first and last name, date of birth, unique patient identifier from time of collection, such as medical record number) on the specimen container and on the test requisition form.
Research or surveillance specimens may be labeled according to protocol. Labels should not include personally identifiable information. The results reported should NOT be used for diagnosis, treatment, assessment of health or management of the individual patient.
Shipping Instructions which Include Specimen Handling Requirements
Methodology Real-time polymerase chain reaction
Turnaround Time 5 Days
Interferences & Limitations Cotton swabs and swabs in media designed for bacterial preservation and/or transport may cause PCR inhibition and should not be used. Specimens with insufficient human DNA will be resulted as inconclusive.
Additional Information Submitters should contact the Poxvirus Inquiry Line by telephone prior to using email and/or contacting the second POC.
Diagnostic real-time polymerase chain reaction can detect the following poxviruses: variola, monkeypox, vaccinia, orf, pseudocowpox, and bovine papular stomatitis virus.
Research real-time polymerase chain reaction can detect the viruses listed above plus cowpox, sealpox, molluscum contagiosum, and tanapox virus.
CDC Points of Contact
Monkeypox virus, Variola virus, Vaccinia virus, smallpox, sore mouth
CDC Pre-Approval Needed
Supplemental Information Required Consultation is required prior to specimen submission. A brief written clinical summary with pertinent medical information (e.g. rash onset date, rash type, symptoms, smallpox vaccination date if relevant) and exposure history should be included. Information must be documented in written form, discussion during initial phone consultation is not a suitable alternative to a written record.
Supplemental Form None
Performed on Specimens from Human and Animal
Acceptable Sample/ Specimen Type for Testing Lesion material is required for persons with an active lesion or rash. A dry swab of lesion fluid or the surface of the lesion is acceptable. If monkeypoxvirus is not suspected, viral culture can also be tested.
Minimum Volume Required 0.5 mL (for viral cultures, if monkeypoxvirus is not suspected)
Collection, Storage, and Preservation of Specimen Prior to Shipping Freeze (-20°C or lower) specimens within an hour after collection. Store frozen samples for up to 60 days. Freezing is strongly recommended. However, if there is no freezer available, refrigerate samples (2-8°C) and store for up to 7 days. Samples must be received within 60 days if frozen or 7 days if refrigerated.
Transport Medium Transport media should not be added to specimens.
Specimen Labeling This test order can accommodate diagnostic and non-diagnostic specimens. Testing subject to CLIA regulations requires two primary patient identifiers (e.g., patient first and last name, date of birth, unique patient identifier from time of collection, such as medical record number) on the specimen container and on the test requisition form.
Research or surveillance specimens may be labeled according to protocol. Labels should not include personally identifiable information. The results reported should NOT be used for diagnosis, treatment, assessment of health or management of the individual patient.
Shipping Instructions which Include Specimen Handling Requirements
CDC does not accept routine shipments on weekends or holidays. Please make sure packages arrive Monday - Friday. Ship specimen(s) refrigerated on cold packs, unless frozen, then ship on dry ice.
Ship to:
<Insert CDC Point of Contact>
Centers for Disease Control and Prevention
RDSB/STATT Unit 47
1600 Clifton Road NE
Atlanta, GA 30329
<Insert CDC Point of Contact's Telephone Number>
All samples must be shipped in accordance with all applicable local, state and federal regulations.
Methodology Real-time polymerase chain reaction
Turnaround Time 5 Days
Interferences & Limitations Cotton swabs and swabs in media designed for bacterial preservation and/or transport may cause PCR inhibition and should not be used. Specimens with insufficient human DNA will be resulted as inconclusive.
Additional Information Submitters should contact the Poxvirus Inquiry Line by telephone prior to using email and/or contacting the second POC.
Diagnostic real-time polymerase chain reaction can detect the following poxviruses: variola, monkeypox, vaccinia, orf, pseudocowpox, and bovine papular stomatitis virus.
Research real-time polymerase chain reaction can detect the viruses listed above plus cowpox, sealpox, molluscum contagiosum, and tanapox virus.
CDC Points of Contact